First causes of respiratory viral infections in children and older adults


High Burden of Disease (BoD) in children and older adults
RSV and HMPV mortality in children mainly in developing countries

Base: highly replicative and hyperfusogenic clinical HMPV strain C-85473 (reverse genetic)
Non-reversible and functional attenuation through deletion of the SH gene
Infectious and replicative in Human Airway Epithelium/low induction of pro-inflammatory cytokines
No pathogenicity or clinical sign in in-vivo ouse model – no enhanced disease
Efficient immune cell recruitment and low inflammatory profile in lung
Protection against HMPV challenge and strong induction of homologous/heterologous neutralizing Ab
Possibility to include other respiratory virus antigens

Cytopathic effects of HMPV at 3 days post-infection (fluorescent microscopy)
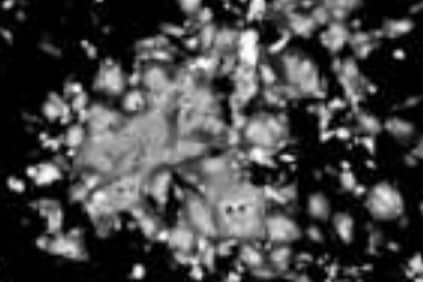
Cytopathic effects of Metavac® at three days post-infection (fluorescent microscopy)
Observation of Metavac® by transmission electron microscopy



HMPV LAV, Infectious, replicative and expressing F-HMPV and F-RSV
Low induction of pro-inflammatory cytokines in Human Airway Epithelium (HAE)
No pathogenicity or clinical sign in murine model - no enhanced disease
Protection against lethal HMPV in-vivo challenge / strong induction of homologous/heterologous NAbs against HMPV-A & B strains
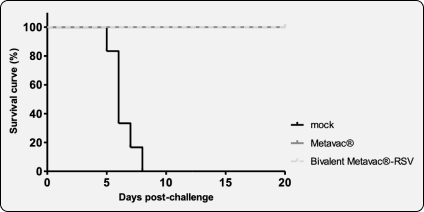
Protection against RSV in-vivo challenge / strong induction of homologous/heterologous NAbs against RSV-A & B strains
Stimulation of mucosal IgA secretionand Th1 cellular response

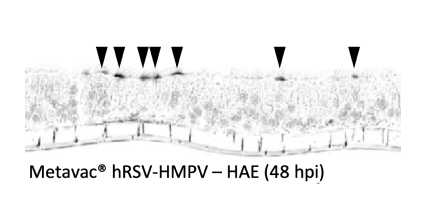
Palivisumab anti-F RSV staining
HMPV challenge
RSV challenge mock-vaccinated

RSV challenge bivalent vaccinated
Fully functional in vitro (human epithelium) and in vivo preclinical models
Infectious and efficiently replicative
Ongoing Process optimistion
No deleterious mutation (antigenic, polymerase) after 10 passages
Basic formulation was validated for 6 months stability (complete preservation of infectious titer at 4°C)

46,000,000
Children below 5 years (EU + US)

151,000,000
older adults above 65 (EU + US)